The European Joint Programme on Rare Diseases (EJP RD) brings over 130 institutions (including all 24 ERNs) from 35 countries:
26 EU Member States (Austria, Belgium, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Croatia, Ireland, Italy, Netherlands, Latvia, Lithuania, Luxembourg, Malta, Poland, Portugal, Romania, Spain, Sweden, Slovakia, Slovenia)
7 associated (Armenia, Georgia, Israel, Norway, Serbia, Switzerland, Türkiye)
Uk & Canada
to create a comprehensive, sustainable ecosystem allowing a virtuous circle between research, care and medical innovation.
As recognized by the Council Recommendation 2009/C 151/02, rare diseases (RD) are a prime example of a research area that can strongly profit from coordination on a European and international scale. RD research should be improved to overcome fragmentation, leading to efficacious use of data and resources, faster scientific progress and competitiveness, and most importantly to decrease unnecessary hardship and prolonged suffering of RD patients.
In the specific context of the massive generation, need for reuse and efficient interpretation of data, introduction of omics into care practice and the structuration of RD care centers in European Reference Networks, it appears crucial and timely to maximize the potential of already funded tools and programmes by supporting them further, scaling up, linking, and adapting them to the needs of end-users through implementation tests in real settings. To achieve this goal, the EJP RD has two major objectives:
To improve the integration, the efficacy, the production and the social impact of research on RD through the development, demonstration and promotion of Europe/world-wide sharing of research and clinical data, materials, processes, knowledge and know-how
To implement and further develop an efficient model of financial support for all types of research on RD (fundamental, clinical, epidemiological, social, economic, health service) coupled with accelerated exploitation of research results for benefit of patients.
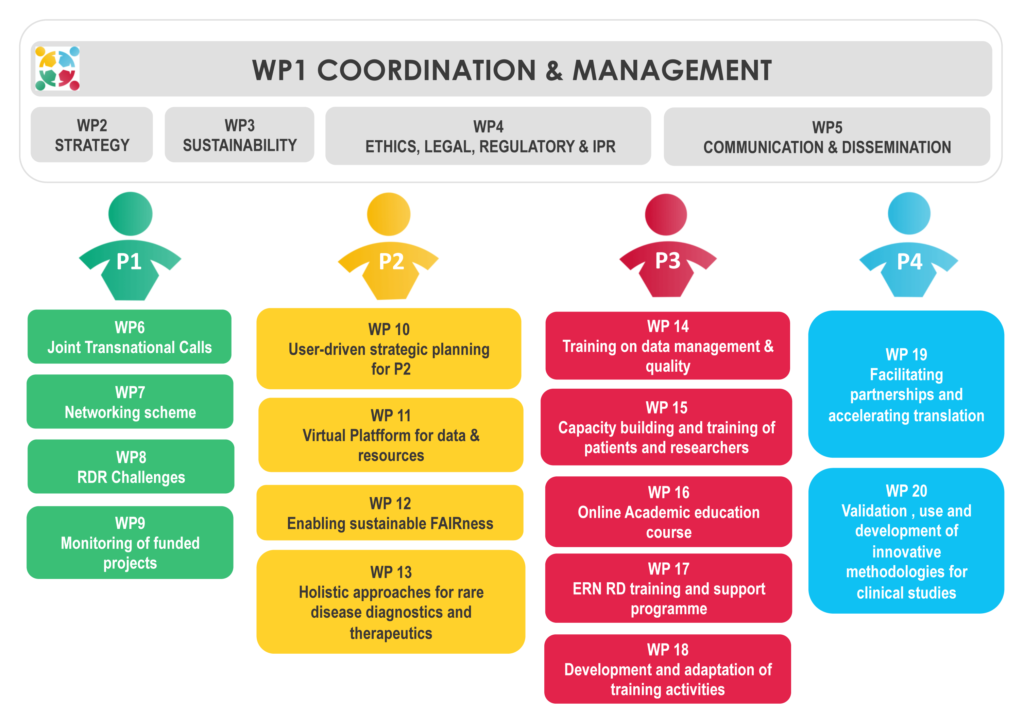
To this end, the EJP RD actions are organized within five major Pillars assisted by the central coordination and transversal activities:
Pillar 0: Coordination, Transversal Activities & Communication
Pillar 1: Fundings and Calls
Pillar 2: Coordinated Access to Data and Services
Pillar 3: Training and Empowerment
Pillar 4: Innovation and Clinical Trials Support

Joanne Lee
Rare Disease Project Manager

Volker Straub
Harold Macmillan Professor of Medicine

Vicki Hedley
Rare Disease Policy Manager