Clinical Research
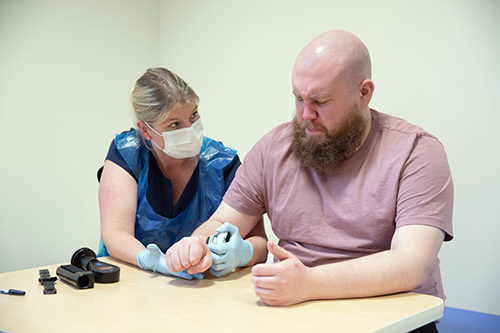
With almost 20 years of experience in delivering clinical research, the John Walton Muscular Dystrophy Research Centre (JWMDRC) is an internationally recognised leader in neuromuscular clinical research.
Aligned with the JWMDRC mission, the clinical research team delivers high standard projects, including observational and natural history studies, interventional trials with advanced therapies, and investigational drugs and devices. We aspire to accelerate translational research in neuromuscular diseases and provide access to innovative and advanced therapies to patients with a broad range of neuromuscular diseases, across paediatric and adult patient populations.
The Clinical Research team includes neuromuscular clinicians, expert physiotherapists, research nurses, project managers, clinical trial coordinators, data managers and clinical trial and project assistants.
Our priorities are clinical research studies focused on advanced therapies and innovative approaches, academic collaborations to advance care, translational research and treatment options targeting patient populations or areas of specific unmet needs.
Integrating research in clinical care
As a translational research centre the insights we gain from both clinical practice and research activity continuously feed into one another ensuring we maintain strong evidence based practice. Within the clinical strand our clinicians are continuously striving to improve patient care and experiences through developing, implementing and offering new research to our patients.
We currently deliver several research studies within the clinical service including the Creatine D3 trial (link), Adult SMA and the AFO-CCD study (static night ankle foot orthosis vs contracture control devices). We also recruit and collect samples for The John Walton Muscular Dystrophy Research Centre Biobank of rare, inherited neuromuscular and neurodegenerative diseases.

Clinical Research Studies
Based on our clinical trial and neuromuscular expertise, we are the national leading site for several academic and commercial clinical research studies and trials. This includes the first gene therapy studies in Duchenne Muscular Dystrophy and Pompe disease coming to the UK.
Clinical studies and trials run at the JWMDRC
Clinical Research projects led by the JWMDRC
We have been leading national and international clinical research projects and collaborations aiming to advance diagnosis, standards of care development, outcome measures and drug development for patients with neuromuscular diseases including:

Adult SMA REACH is a data collection study aiming to gain a better understanding of the impact of standards of care and new treatments on the natural history of Spinal Muscular Atrophy (SMA).

Myo-Guide is an artificial intelligence (AI) based algorithm that supports the diagnosis of patients with neuromuscular diseases through an analysis of the pattern of fat replacement on muscle MRI.

The International Clinical Outcome Study for Dysferlinopathy (COS) has been running since September 2012 with the generous support of the Jain Foundation.

The BIND study aims to improve the learning and behavioural challenges in individuals with DMD or BMD.

This pilot study aimed to identify the sample size required to power a larger scale study to compare AFOs and CCDs in managing ankle range of movement and function in boys with DMD. It will also explore adherence and patient satisfaction for the two devices.

This study aims to address the critical need for early detection and treatment monitoring in LOPD by employing a new MRI technique, Carbon Spectroscopy, which detects the Carbon present in the glycogen of the muscles and therefore allows us to measure glycogen levels in patients.

This centre aims to further neuromuscular research in partnership by building diverse genetically diagnosed international cohorts, increasing access to genomic medicine and clinical trials and importantly investing in training the next generation of NMD researchers.

The FOR DMD study was an international, double blind, controlled study which has been established to assess the relative effectiveness and adverse event profiles of the 3 most frequently prescribed corticosteroid regimes in DMD.
Participation in Clinical Trials
The Patient Journey
We are involved in clinical trials and research projects in different neuromuscular diseases across the full life span, from paediatric to adulthood.
Equitable/fair access to clinical research is our priority. Patients are identified through our clinic list but we also receive referrals from other clinical centres and collaborate with patient registries and databases to ensure that access to clinical trials is not limited to patients follow up at our centre.
I am always treated with respect, I feel part of the team
It's great to see people who understand totally, have empathy but still positive. Great to be able to discuss any concerns without seeming silly. Invaluable to have the link with my doctors to get updates and referrals. Cannot fault or undervalue the importance of how the research has helped me and my family
The whole experience has been life changing, from the care given to the people I have met and kept contact with. I feel far more connected to those researching my condition and a deep understanding of how this is progressing
Locations
Our research study and clinical trial activity is delivered at specialist centres within the Newcastle University NHS Trust at locations across Newcastle City Centre.
Meet the Clinical Research team

The Project Management Team
EXPERTISE:
Expertise in all phases of project management, from project initiation, planning, and execution to monitoring and closure for a variety of research projects.
Natural history studies, to understand the evolution of neuromuscular diseases.
Observational studies of Real-World data collection for regulatory approval of new treatments.
MRI studies as a biomarker/outcome measure to assess disease progression.
Delivery of international project and collaborative studies lead by the John Walton Muscular Dystrophy centre.
Delivery of studies applying AI to improve diagnosis and care.
Grant application support
Expertise in long term planification of research projects

Jess Page
Project Manager

Sam Fitzsimmons
Project Manager

Alejandro Gonzalez Chamorro
Project Manager

Heather Hilsden
Jain Foundation Project Manager

Claire Robinson
BRC Project Manager
Stephen Wandera
Data Quality Assurance Manager

The Clinical Trial Coordination team
EXPERTISE:
Set up of clinical research studies, ranging from observational studies through the clinical trials of investigational medicinal products to advanced therapies in a broad spectrum of neuromuscular diseases
Support for advanced therapy studies
Set up and delivery of international multi-centre clinical trials
Grant applications support
Development of and support for the implementation of national guidance on costing for clinical trial in neuromuscular diseases

Ellie Drummond
Clinical Trial Coordinator

The Research Nurses
EXPERTISE:
Provide ongoing support and information to participants on their journey through an academic research study or clinical trial.
As well as delivering the nursing activities required for a study or trial, they will collect, record and verify all study data with a high degree of accuracy to ensure high quality research.
Act in the best interests of the research participants and will oversee and maintain effective communication between the research team and the study participants, their relatives and the multidisciplinary team involved in their care.

Sara Wilkinson
Research Nurse

Elaine Stephenson
Research Nurse
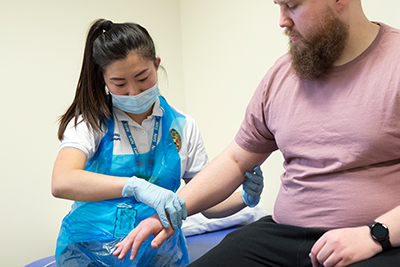
The Physiotherapy team
EXPERTISE:
Leaders in developing clinical outcome assessments used in NMD trials and clinics internationally including the North Star Ambulatory Assessment, Performance of Upper limb 2.0 and North Star Assessment for limb girdle type muscular dystrophies
Advise on trial design and outcome measure selection in natural history studies and clinical trials
Collect primary and secondary endpoints across paediatric and adult natural history studies and interventional trials

Dionne Moat
Neuromuscular Research Physiotherapist

Jassi Michell-Sodhi
Clinical Specialist Physiotherapist

Karen Wong
Neuromuscular Research Physiotherapist

Emma Grover
Neuromuscular Research Physiotherapist

Meredith James
Clinical Specialist Research Physiotherapist

Emma Robinson
Neuromuscular Research Physiotherapist

Michelle McCallum
Physiotherapy Technical Instructor

Robert Muni Lofra
Consultant Physiotherapist

Pete Waldock
Neuromuscular Research Physiotherapist

The Data Management team
EXPERTISE:
Maintenance of national and international patient databases and post-marketing registries
Data quality
Implement innovative methods to monitor large data sets
Data quality
Implement innovative methods to monitor large data sets

Elena Karkkainen
Data Manager

Jamie Mitchell
Data Manager
PhD Students
Current PhD students include:
-
"The role of magnetic resonance imaging in the study of dysferlinopathies"

Carla Bolaño Diaz
-
"Design and implementation of a fully automatic pipeline for muscle MRI segmentation, fat fraction, quantification and neuromuscular disease diagnostics"

Jose Verdú-Díaz
-
"The results of the FOR DMD study and the impact of early corticosteroids in DMD: implications for clinical care and clinical trial design"

Marianela Schiava
-
University of Padova: "Translational Specialistic Medicine: Dystrophinopathies - deep phenotyping"

Pietro Riguzzi
Collaborations
We have a well-established framework working together with the other strands at the JWMDRC such as the Clinical Team, Basic Research Team, Networking Team as well as the The John Walton Muscular Dystrophy Research Centre Biobank. We have strong collaborations with both academic partners, pharmaceutical companies, national and international networks as well as disease registries, patient organisations and advocacy groups.
DMD Hub
We are the coordinating centre for the DMD Hub and one of the leading DMD Hub trial site. Our team members are members of the DMD Hub networks (clinical trial coordinators, research nurses, Principal Investigators, young investigators) and actively contribute to the development of resources to support other clinical trial sites and promoting harmonisation of clinical trial conduct in the UK.
Biobank

An integral part of our clinical and clinical research activities is the collection of samples (blood, urine, saliva, muscle biopsy, skin biopsy, etc.) for the JWMDRC Research Biobank to be used in research projects across the world.
The Northern Alliance Advanced Therapies Treatment Centre
We are part of The Northern Alliance Advanced Therapies Treatment Centre (NAATTC), a consortium of twenty industry, NHS and academic organisations led by Newcastle Hospitals and Scottish National Blood Transfusion Service (SNBTS). The purpose of the NATTC is to develop the systems and infrastructure required to support the delivery of cell and gene therapies with the ultimate aim of increasing patient access to advanced therapy medicinal products (ATMPs) on a national level.
Biomedical Research Centre
We are part of the National Institute for Health and Care Research (NIHR) Newcastle Biomedical Research Centre (BRC) which brings together world-leading researchers and clinicians to turn new scientific breakthroughs into treatments for patients.
Our centre is a co-lead on the Neuromuscular Disease, Rare Diseases and Mitochondrial Dysfunction theme. Our aim is to systematically develop and evaluate novel approaches to diagnosing, monitoring and treating neuromuscular disease (NMD) and mitochondrial dysfunction, and to extend the application of these technologies across the broader spectrum of rare diseases.
Northstar Clinical Network
We are proud to be active collaborators in the North Star Clinical Network.
The North Star Project was set up in 2003 to help drive improvements in services and set national standards of care for children living with Duchenne Muscular Dystrophy (DMD). The overall aim is to optimise the care of young patients with Duchenne Muscular Dystrophy (DMD) by achieving and practising consensus on best clinical management, with agreed assessment and treatment protocols, irrespective of the treating clinical centre.
The project aims to:
Provide best support to patients by agreeing protocols for assessment and best practice treatment options
Assist clinicians working with muscle disease by developing a national clinical network and providing a discussion forum to promote best patient care
Standardise and optimise steroid therapy in ambulant children with DMD throughout the UK
Ensure a standard assessment protocol for newly diagnosed children and those due to start corticosteroid treatment

Working with patient organisations
We work extensively in partnership with patient organisations nationally and internationally to drive research, improve care and engage with the neuromuscular community. We are always pleased to discuss opportunities for collaboration and discuss your priorities.
“We have been working closely with the team at the John Walton Muscular Dystrophy Research Centre since 2012 on the International Clinical Outcome Study for Dysferlinopathy.
Hundreds of genetically confirmed dysferlinopathy patients have participated globally in COS and COS2 as well as over 135 clinicals and medical staff. A total of 1248 clinic visits were completed world wide. The high level of patient retention is an important measure of their success.“
Sarah Shira Emmons VP Patient Affair, The Jain Foundation

